
The atomic number is the total number of protons present in the nucleus of an atom. The sum of protons and neutrons gives the atomic mass of an element. The nucleus is surrounded by negatively charged electrons. Neutrons are neutral so that it doesn’t have any charge on them. The centre of the atom is also called the nucleus which is positively charged and consists of protons and neutrons. It consists of electrons, protons and neutrons. An atom is the smallest indivisible unit of matter. An element is made up of a single type of atom. The concept of atomic number and Valency can only be understood if you know what exactly elements are made up of. It is important to know the atomic number and electronic configuration of an element to find its valency. The valency of an element is determined by the number of electrons in the valence shell. The number of electrons in the outermost shell is called valence electrons and the outermost shell is called the valence shell. This ability of an atom to gain or lose electrons to achieve a stable configuration or inert gas configuration is called the Valency of an element.

of a noble gas, the atom of an element tries to gain or lose electrons. In order to achieve the most stable configuration i.e. Without the symbols, it would have been a herculean task to represent all these 118 elements and the umpteen numbers of compounds they form.

Chemical formulas and equations are also represented using those symbols. For example, the element with atomic number 110 was named as ‘un un nilium’ with the symbol 'Uun', now it is named Ds.Īs far as students are concerned, it is important to study all the 118 elements with their Symbol and Valency. The elements which are new are temporarily named according to their atomic numbers. When the symbol representing an element is denoted by one letter only, it is written in uppercase.Įxample: 'N' represents Nitrogen, 'O' represents oxygen, etc. Example: 'Ca' representing Calcium, 'He' representing Helium, etc. The first letter of a symbol is capitalized with the second (or third) letters being in lowercase. Rules or Conventions followed to denote the Element using Symbol The symbol 'Fe' is used to denote Iron, as the Latin word for Iron is "Ferrum". Hence gold is denoted by the symbol ‘Au’. Some symbols of a few elements are derived from their Latin or Greek names. One may ask, ‘How is the symbol of an element derived?’ We can see in the table above that most of the symbols are derived from the elements’ names, by taking either the first or first two letters from the English name of the element. Some symbols have three letters, they generally represent synthesized elements newly, with some being temporarily named like that. Example: Elements in group 1A are soft metals that react violently with water.Ī symbol representing a chemical element is a 'sign' or 'notation' that generally consists of one or two letters.
Elements arranged vertically in columns are called ‘Groups’ and elements arranged horizontally in rows are called ‘Periods’.įurther elements are grouped as per periodic trends and properties.
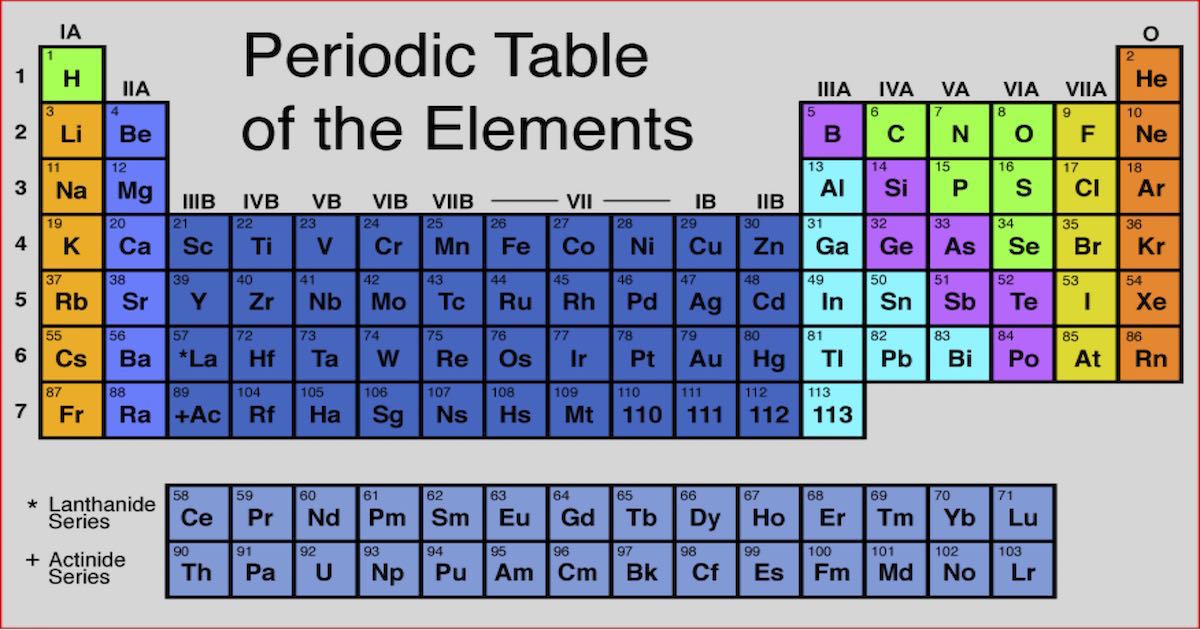
Key Characteristics of the Periodic Table:Įlements are arranged in order of increasing atomic number.Įlements of the Periodic Table are denoted by a unique symbol and not its entire name, as some elements’ names can be long and complex in nature.Įlements are arranged vertically and horizontally.


 0 kommentar(er)
0 kommentar(er)
